Metina has internal experts for review and writing of biosimilar application pertaining to USA, EU, BRICS-TM markets and WHO.

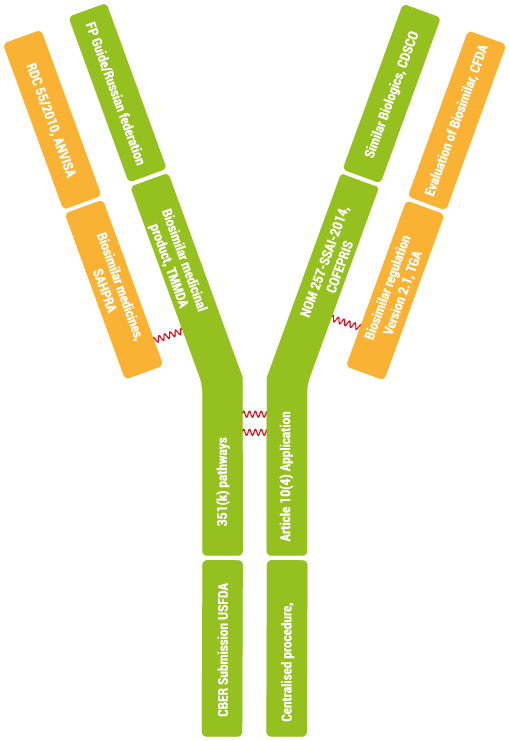
Metina has internal experts for review and writing of biosimilar application pertaining to USA, EU, BRICS-TM markets and WHO.