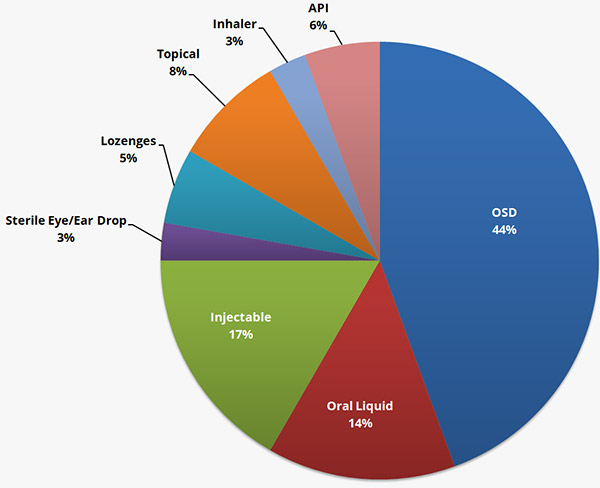
Metina has efficiently managed multiple ANDAs submissions and approvals. The assistance was given starting from regulatory advice during product development, controlled correspondence with FDA, writing of scientific dossier in CTD, conversion into eCTD, online submission support to USFDA, query response and product approval.