
- Quality and Scientific Regulatory Services for Global Bio-/Pharmaceutical Industry
Biosimilars: Getting the competitive edge
- Home
- Biosimilar Intelligence
- Biosimilars: Getting the competitive edge
- 0 Comments
Biosimilars are ‘blockbuster drugs’ or the ‘affordable copies’ of expensive innovator products which are instrumental in reducing healthcare costs as well as improving patient access to important, lifesaving medications. They are biological products highly similar to the reference product in terms of safety, purity and potency, but may have minor differences in clinically inactive components. Developing a biosimilar involves complex manufacturing process and involves demonstration of quality, efficacy and safety of the product and most importantly, proving similarity to the reference product.
An exciting, growing market…
The biosimilars market is fast-moving and evolving rapidly, with an ever-changing regulatory landscape in both emerging and developed markets. The global biosimilars market is expected to exceed more than US$ 15.0 million by 2024 out of which 40% is occupied by the Rest-of-World. Despite presence of defined biosimilar regulatory pathways in many emerging markets, they are still in flux in high profile countries like BRICS-TM and others. Hence, it becomes essential for the companies to strategize on the intended potential markets, and further expand to other regions. Finding the right path may be risky and being updated with current information is key for such strategies. Further, the pace of change in regulatory norms also requires optimal designing of clinical programs in line with the latest requirements in targeted markets.
Quick solution, quick access…
Understanding the intended biosimilar product is crucial for effective development of the product. Using the right expertise for a focused and efficient biosimilar development program and an expedited approval pathway will save time and money.
Key challenges…
- Regulatory strategy
- CMC comparability plan
- Manufacturing strategy
- Development and validation of analytical methods and bioassays
- Comparative non-clinical/ clinical development plan
- Pricing strategy
“Do you wish to see yourself in biosimilar space? Are you aware of specific obstacles and strategies to bring your product to market?”
Put our biosimilar expertise to work for you!!!
Metina PharmConsulting’s 5Es to see your biosimilar product in the market earlier than before.
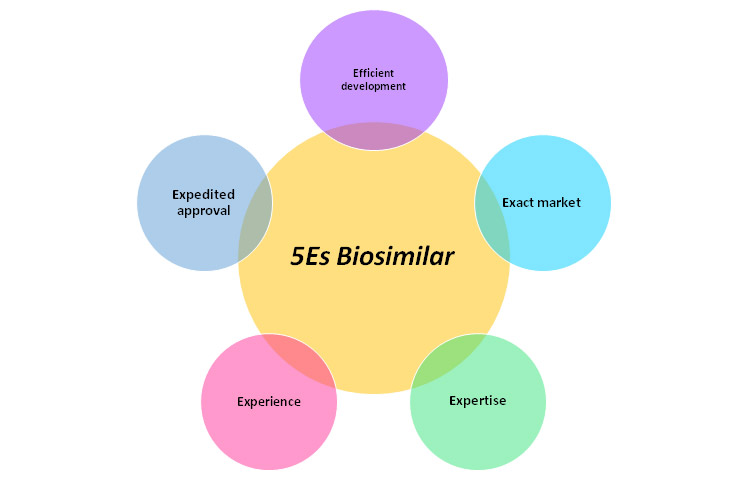
Metina offers end-to-end regulatory service and product development consulting services for biosimilar and other biological products for CMC, Nonclinical and Clinical development.
We have a specialized team of experts with core biopharmaceutical industry experience, with in-depth understanding of biosimilar development processes & regulatory requirements at different phases of product life cycle.
With a strong connect for regulatory and marketing task across BRICS-TM countries, Asia, Africa (Including MENA), GCC and Latin America, we can get your product faster in the hands of patients.
Services offered:
- Regulatory strategy: Developing regulatory strategies for international markets/global submissions.
- Product registration: Regulatory authoring, review, compilation and submissions (ICH CTD, e-CTD, ASEAN CTD, Country-specific dossier, Technology transfer package) for:
- India: RCGM submissions, CTA submission, MAA submission
- ROW/ Emerging market: LATAM, BRICS-TM, ASEAN, CIS, ME, PIC/S
- Addressing deficiency responses from Health authorities